import numpy as np
from numpy import linalg as LAcore
Fill in a module description here
Unit
q1 = Q(-75, 'ncoulomb')Summary
Key ideas: - Electron energy levels are discrete - Ionization energy
The energy emits when electron make transition between energy levels
Rydberg10973731.56816lambdaa, nu = smp.symbols('lambda nu')lambdaalambdanunun_i, n_f = smp.symbols('n_i n_f')n_in_iformula = (Rydberg*h*c) * ((1/n_i**2) - (1/n_f**2))formula2.17987236110358e-18/n_i**2 - 2.17987236110358e-18/n_f**2eq = smp.Eq(1/lambdaa, formula)eqEq(1/lambda, 2.17987236110358e-18/n_i**2 - 2.17987236110358e-18/n_f**2)formula.subs(1, 2).evalf()2.17987236110358e-18/n_i**2 - 2.17987236110358e-18/n_f**2# #| export
# def the_wavelength_emit_from_transition(n1: 'the initial energy level', n2: 'the final energy level'):
# formula = (Rydberg*h*c) * ((1/n_i**2) - (1/n_f**2))
# formula_evaled = formula.subs([(n_i, n1), (n_f, n2)]).evalf()
# equation = smp.Eq(1/lambdaa, formula_evaled)
# return smp.solve(equation, lambdaa)#energy_emit_from_transition(2, 1)# the_wavelength_emit_from_transition(2, 1)The energy that a photon carries
h6.62607015e-34c299792458.0\[\mathrm{E}=h \nu\] \[E=\frac{h c}{\lambda}\]
calculate_energy
calculate_energy (**kwargs)
h6.62607015e-34c299792458.0h*c1.9864458571489286e-25calculate_energy(wavelength=525)3.783706394569388e-28calculate_energy(wavelength=3.37e-7)5.894498092430055e-19def calculate_wavelength_from_energy(energy):
return energy/hdef calculate_wavelength_from_wavelength(frequency):
passcalculate_energy(wavelength=2)9.932229285744643e-26Emission Spectrum
Use energy transitions to characterize materials
Bond
- Shell: like to be
total filled>totally empty>partial filled
Oxidation state of an atom tells
from mendeleev import Si, Fe, O, Al, Ca, Ti, F, Cr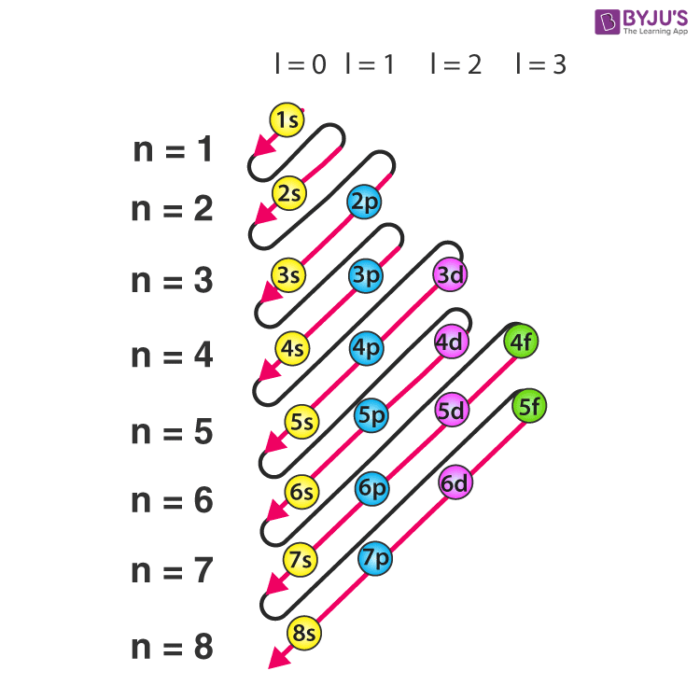
Si.name'Silicon'Al.oxistates[3]Ca.oxistates[2]Ti.oxistates[4, 3, 2]F.oxistates[-1]Cr.oxistates[6, 3, 2]Cr.ec.confOrderedDict([((1, 's'), 2),
((2, 's'), 2),
((2, 'p'), 6),
((3, 's'), 2),
((3, 'p'), 6),
((3, 'd'), 5),
((4, 's'), 1)])Fe.ec.confOrderedDict([((1, 's'), 2),
((2, 's'), 2),
((2, 'p'), 6),
((3, 's'), 2),
((3, 'p'), 6),
((3, 'd'), 6),
((4, 's'), 2)])Fe.oxistates[3, 2]from mendeleev import NaNa.oxistates[1]